Chemistry, 09.10.2019 04:30 richardgibson2005
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbon dioxide (co2), and water (h2o). a chemist carries out this reaction in a bomb calorimeter. the reaction causes the temperature of a bomb calorimeter to decrease by 0.985 k. the calorimeter has a mass of 1.500 kg and a specific heat of 2.52 j/g•k. what is the heat of reaction for this system?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbo...
Questions


Biology, 06.05.2020 21:41



Mathematics, 06.05.2020 21:41

Mathematics, 06.05.2020 21:41


English, 06.05.2020 21:41

Mathematics, 06.05.2020 21:41


English, 06.05.2020 21:41

Mathematics, 06.05.2020 21:41

Biology, 06.05.2020 21:41







Mathematics, 06.05.2020 21:41

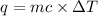
 = change in temperature = 0.985 K
= change in temperature = 0.985 K