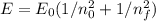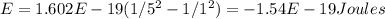Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1


Chemistry, 23.06.2019 17:00
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1
You know the right answer?
Calculate the energy of a photon emitted when an electron in a hydrogen atom undergoes a transition...
Questions


History, 28.05.2020 02:02

Mathematics, 28.05.2020 02:02

Mathematics, 28.05.2020 02:02


Mathematics, 28.05.2020 02:02






Biology, 28.05.2020 02:02



Mathematics, 28.05.2020 02:02




Chemistry, 28.05.2020 02:02

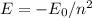 , where E = 1.602E-19 Joules.
, where E = 1.602E-19 Joules.