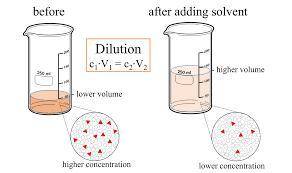
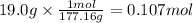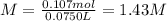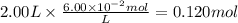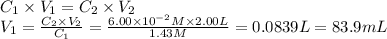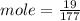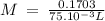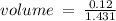On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by adding 19.0g of luminol into a total volume of 75.0ml of h2o.
a)what is the molarity of the stock solution of luminol?
anwer i got: molarity of luminol solution = 1.43m b)before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00×10−2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces.
i cannot get the correct answer for "c" have tried: 172ml,11.9ml, and 1.19*10^4. the only other possibility that i can come up with is: 83.9ml. would this one be i still completely out to
c)how many moles of luminol are present in 2.00 l of the diluted spray?
anwer i got: moles of luminol = 0.120mol what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?
express your answer in milliliters.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1


Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

You know the right answer?
On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by...
Questions

History, 23.08.2021 22:50




Chemistry, 23.08.2021 22:50

Biology, 23.08.2021 22:50





Mathematics, 23.08.2021 22:50



Mathematics, 23.08.2021 22:50


Mathematics, 23.08.2021 22:50


Mathematics, 23.08.2021 22:50

Mathematics, 23.08.2021 22:50

Mathematics, 23.08.2021 22:50