
Chemistry, 15.10.2019 01:30 Justinoreilly71
The reason iodine, i2, exists as a solid at room temperature, while bromine, br2, exists as a liquid at the same temperature, is because:
iodine exhibits hydrogen bonding, while bromine exhibits only london dispersion forces.
iodine molecules create stronger london dispersion forces than do bromine molecules.
bromine exists only as a gas at all temperatures.
bromine forms fewer dipole-dipole interactions than does iodine.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
The reason iodine, i2, exists as a solid at room temperature, while bromine, br2, exists as a liquid...
Questions

Mathematics, 20.05.2021 17:50


Arts, 20.05.2021 17:50




Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50




Social Studies, 20.05.2021 17:50


Mathematics, 20.05.2021 17:50

History, 20.05.2021 17:50




Mathematics, 20.05.2021 17:50

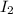 , exists as a solid at room temperature, while bromine,
, exists as a solid at room temperature, while bromine, 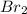 , exists as a liquid at the same temperature, is because: Iodine molecules create stronger London dispersion forces than do bromine molecules.
, exists as a liquid at the same temperature, is because: Iodine molecules create stronger London dispersion forces than do bromine molecules.

