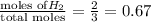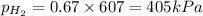Abox with a volume of 11.2 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0°c which of the following statements is true?
r = 8.31 kpa x l/mol x k, pv = nrt
a. the partial pressures of n₂ and h₂ are equal.
b. the partial pressure of n₂ is 202 kpa.
c. the total pressure is 303 kpa.
d. the total pressure in the box is 202 kpa.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
Abox with a volume of 11.2 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0°c which of th...
Questions


Mathematics, 15.12.2019 15:31


Biology, 15.12.2019 15:31

English, 15.12.2019 15:31

Mathematics, 15.12.2019 15:31


Mathematics, 15.12.2019 15:31




Mathematics, 15.12.2019 15:31

History, 15.12.2019 15:31

Mathematics, 15.12.2019 15:31


Geography, 15.12.2019 15:31


Business, 15.12.2019 15:31

Mathematics, 15.12.2019 15:31

Chemistry, 15.12.2019 15:31

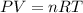
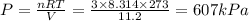
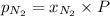
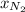 = mole fraction=
= mole fraction= 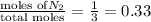
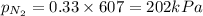
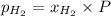
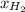 = mole fraction=
= mole fraction=