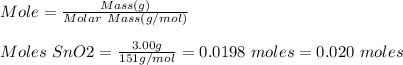Chemistry, 03.02.2020 21:02 alyssamaize
Base your answer to the question on the information below and on your knowledge of chemistry. at 1023 k and 1 atm, a 3.00-gram sample of sno2(s) (gram formula mass = 151 g/mol) reacts with hydrogen gas to produce tin and water, as shown in the balanced equation below. sno2(s) + 2h2(g) → sn(l) + 2h2o(g) show a numerical setup for calculating the number of moles of sno2(s) in the 3.00-gram sample.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
Base your answer to the question on the information below and on your knowledge of chemistry. at 102...
Questions

Mathematics, 06.01.2020 14:31

Mathematics, 06.01.2020 14:31

Mathematics, 06.01.2020 14:31


Physics, 06.01.2020 14:31




Biology, 06.01.2020 14:31


Mathematics, 06.01.2020 14:31


Mathematics, 06.01.2020 14:31

History, 06.01.2020 14:31


Mathematics, 06.01.2020 14:31


English, 06.01.2020 14:31