
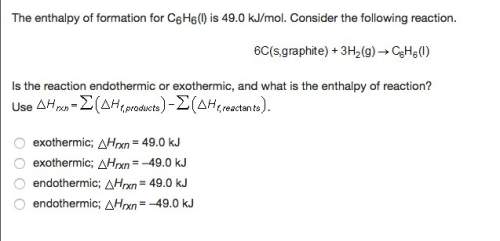
The enthalpy of formation for c6 h6 (i) is 49.0 kj/mol. consider the following reaction.
is the reaction endothermic or exothermic, and what is the enthalpy of reaction?
exothermic; mc014-3.jpghrxn = 49.0 kj
exothermic; mc014-4.jpghrxn = –49.0 kj
endothermic; mc014-5.jpghrxn = 49.0 kj
endothermic; mc014-6.jpghrxn = –49.0 kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
The enthalpy of formation for c6 h6 (i) is 49.0 kj/mol. consider the following reaction.
...
...
Questions


History, 04.08.2019 18:50


Spanish, 04.08.2019 18:50


History, 04.08.2019 18:50


History, 04.08.2019 18:50


Geography, 04.08.2019 18:50

Geography, 04.08.2019 18:50

History, 04.08.2019 18:50




Mathematics, 04.08.2019 18:50

Chemistry, 04.08.2019 18:50

Health, 04.08.2019 18:50

History, 04.08.2019 18:50

Social Studies, 04.08.2019 18:50

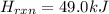
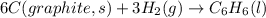
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0345/6718/76c37.png)
![\Delta H=[(n_{C_6H_6}\times \Delta H_{C_6H_6})]-[(n_{H_2}\times \Delta H_{H_2})+(n_{C}\times \Delta H_{C})]](/tpl/images/0345/6718/5d909.png)
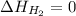 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
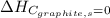 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
![\Delta H=[(1\times 49.0)]-[(3\times 0)+(6\times 0]](/tpl/images/0345/6718/8bb49.png)
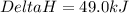
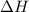 for the reaction comes out to be negative.
for the reaction comes out to be negative.



