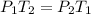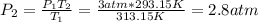Chemistry, 22.09.2019 22:00 Idontknow708
If the moles and volume of a gas are held constant, dropping the temperature from 40 degrees celsius to 20 degrees celsius causes would cause an initial pressure of 3 atmospheres to drop to 1.5 atmospheres.
t/f

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 07:20
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3

Chemistry, 23.06.2019 12:30
The equilibrium constant kc for the reaction 2 nocl(g) → 2 no(g) + cl2(g) is 0.453 at a certain temperature. a mixture of nocl, no, and cl2 with concentrations 1.30, 1.20, and 0.600 m, respectively, was introduced into a container at this temperature. which of the following is true? 1. no apparent reaction takes place. 2. [cl2] = 0.30 m at equilibrium. 3. nocl(g) is produced until equilibrium is reached. 4. [nocl] = [no] = [cl2] at equilibrium. 5. cl2(g) is produced until equilibrium is
Answers: 3

You know the right answer?
If the moles and volume of a gas are held constant, dropping the temperature from 40 degrees celsius...
Questions

History, 12.12.2020 17:00


English, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Physics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00


Chemistry, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Health, 12.12.2020 17:00

Social Studies, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00