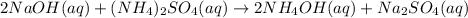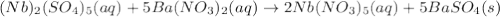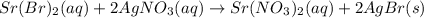Chemistry, 06.10.2019 13:00 genyjoannerubiera
Predict whether each of the following reactions will occur in aqueous solutions. explain your reasoning in each case. note: barium sulfate and silver bromide precipitate in aqueous solutions.
(a) sodium hydroxide + ammonium sulfate → ?
(b) niobium(v) sulfate + barium nitrate → ?
(c) strontium bromide + silver nitrate → ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

You know the right answer?
Predict whether each of the following reactions will occur in aqueous solutions. explain your reason...
Questions

Business, 29.10.2020 18:00


Mathematics, 29.10.2020 18:00



Mathematics, 29.10.2020 18:00

Mathematics, 29.10.2020 18:00

Mathematics, 29.10.2020 18:00

Health, 29.10.2020 18:00




English, 29.10.2020 18:00



History, 29.10.2020 18:00

Mathematics, 29.10.2020 18:00


Mathematics, 29.10.2020 18:00

Mathematics, 29.10.2020 18:00