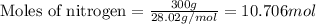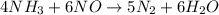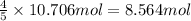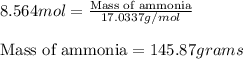Chemistry, 14.10.2019 21:40 WindelCaceus123
In the following reaction, how many grams of ammonia (nh3) will produce 300 grams of n2? 4nh3 + 6no → 5n2 + 6h2o the molar mass of ammonia is 17.0337 grams and that of nitrogen is 28.02 grams.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
In the following reaction, how many grams of ammonia (nh3) will produce 300 grams of n2? 4nh3 + 6no...
Questions

Mathematics, 05.05.2020 07:51





Mathematics, 05.05.2020 07:51


Mathematics, 05.05.2020 07:51





Social Studies, 05.05.2020 07:51

History, 05.05.2020 07:51


Social Studies, 05.05.2020 07:51

Mathematics, 05.05.2020 07:51

Chemistry, 05.05.2020 07:51

Mathematics, 05.05.2020 07:51

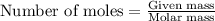 ....(1)
....(1)