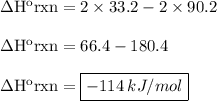Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1

Chemistry, 23.06.2019 15:30
How many moles of potassium nitrate, kno3 are present in a sample with a mass of 85.2 g?
Answers: 1
You know the right answer?
Calculate the enthalpy of the reaction 2no(g)+o2(g)→2no2(g)
1/2n2(g)+o2(g)→no2(g), δh∘a=33.2...
1/2n2(g)+o2(g)→no2(g), δh∘a=33.2...
Questions

Mathematics, 15.12.2019 06:31

Mathematics, 15.12.2019 06:31

Mathematics, 15.12.2019 06:31



Physics, 15.12.2019 06:31

Mathematics, 15.12.2019 06:31

English, 15.12.2019 06:31

Social Studies, 15.12.2019 06:31




Chemistry, 15.12.2019 06:31

Biology, 15.12.2019 06:31

Social Studies, 15.12.2019 06:31

Mathematics, 15.12.2019 06:31


History, 15.12.2019 06:31

Business, 15.12.2019 06:31

Mathematics, 15.12.2019 06:31