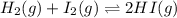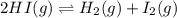Chemistry, 19.11.2019 17:31 mattydoug4818
Two experiments were performed involving the following equilibrium. the temperature was the same in both experiments. h2(g) + i2(g) 2hi(g) in experiment a, 1.0 m i2 and 1.0 m h2 were initially added to a flask and equilibrium was established. in experiment b, 2.0 m hi was initially added to a second flask and equilibrium was established. which of the following statements is always true about the equilibrium concentrations?
a.[h2] equals [hi] in experiment a.
b.[hi] equals 2[h2] in experiment a.
c.[hi] in experiment a equals [hi] in experiment b.
d.[hi] in experiment a equals 1/2[i2] in experiment b.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Two experiments were performed involving the following equilibrium. the temperature was the same in...
Questions


Mathematics, 01.08.2019 09:30


English, 01.08.2019 09:30

History, 01.08.2019 09:30

Biology, 01.08.2019 09:30


Mathematics, 01.08.2019 09:30

Biology, 01.08.2019 09:30

Computers and Technology, 01.08.2019 09:30



History, 01.08.2019 09:30

Mathematics, 01.08.2019 09:30





History, 01.08.2019 09:30