
Chemistry, 18.09.2019 18:00 shelbycg02
What do colligative properties depend on? a) the identity of the solute b) the number of particles present in the solution c) the quantity of solvent in the solution d) none of these

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
What do colligative properties depend on? a) the identity of the solute b) the number of particles...
Questions

Physics, 24.02.2021 15:50

English, 24.02.2021 15:50





Biology, 24.02.2021 15:50



Mathematics, 24.02.2021 15:50

Mathematics, 24.02.2021 15:50

Mathematics, 24.02.2021 15:50

Arts, 24.02.2021 15:50


Mathematics, 24.02.2021 15:50



Mathematics, 24.02.2021 15:50


Mathematics, 24.02.2021 15:50


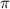 = osmotic pressure
= osmotic pressure


