
Chemistry, 22.11.2019 16:31 angelina12386
Combustion analysis of an unknown compound provides the following data: 73.5 grams c, 4.20 grams h, and 72.3 grams cl. what is the percent composition of each element in this compound?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1
You know the right answer?
Combustion analysis of an unknown compound provides the following data: 73.5 grams c, 4.20 grams h,...
Questions




Mathematics, 12.06.2021 14:00


Mathematics, 12.06.2021 14:00

Mathematics, 12.06.2021 14:00







History, 12.06.2021 14:00


Mathematics, 12.06.2021 14:00

Mathematics, 12.06.2021 14:00

Mathematics, 12.06.2021 14:00

Mathematics, 12.06.2021 14:00

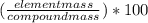
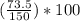 = 49.0%
= 49.0%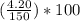 = 2.8%
= 2.8%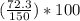 = 48.2%
= 48.2%

