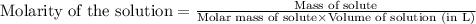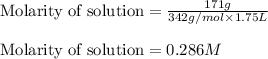Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
There are 342 g of sucrose in 1.00 mol of sucrose. what is the molar concentration (molarity) of a s...
Questions

History, 16.04.2020 22:21

Mathematics, 16.04.2020 22:21


Mathematics, 16.04.2020 22:21

History, 16.04.2020 22:21

Mathematics, 16.04.2020 22:21












Mathematics, 16.04.2020 22:22

Biology, 16.04.2020 22:22

Mathematics, 16.04.2020 22:22