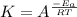a. the pressure of the system decreases.

Chemistry, 18.09.2019 18:00 patelandrew816
What happens as the activation energy increases?
a. the pressure of the system decreases.
b. the kinetic energy of colliding molecules changes.
c. the reactant surface area decreases.
d. the reaction begins to slow down.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
What happens as the activation energy increases?
a. the pressure of the system decreases.
a. the pressure of the system decreases.
Questions


History, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01

English, 18.08.2020 09:01

English, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01



Mathematics, 18.08.2020 09:01

History, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01


Advanced Placement (AP), 18.08.2020 09:01

Mathematics, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01



Mathematics, 18.08.2020 09:01