
Chemistry, 17.11.2019 02:31 aaronw3743
Using the equation, 4fe + 3o2 imported asset 2fe2o3, if 8 moles of iron and oxygen from the air were available, how many moles of iron (iii) oxide would be produced?
4 moles
5 moles
6 moles
8 moles

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
Using the equation, 4fe + 3o2 imported asset 2fe2o3, if 8 moles of iron and oxygen from the air were...
Questions

Mathematics, 07.11.2020 01:00


Mathematics, 07.11.2020 01:00

Social Studies, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00


Health, 07.11.2020 01:00

Biology, 07.11.2020 01:00


Physics, 07.11.2020 01:00


Chemistry, 07.11.2020 01:00





Mathematics, 07.11.2020 01:00


Biology, 07.11.2020 01:00

Arts, 07.11.2020 01:00

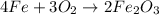
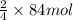 of iron oxide.
of iron oxide.

