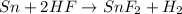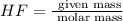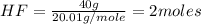Chemistry, 09.10.2019 05:00 onewaydemon
The chemical equation below shows the reaction between tin (sn) and hydrogen fluoride (hf). sn + 2hf snf2 + h2 the molar mass of hf is 20.01 g/mol. how many moles of sn are required to react with 40 g of hf? 1 2 3 4

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
The chemical equation below shows the reaction between tin (sn) and hydrogen fluoride (hf). sn + 2hf...
Questions

English, 07.11.2020 23:10



English, 07.11.2020 23:10


English, 07.11.2020 23:10




Mathematics, 07.11.2020 23:10



English, 07.11.2020 23:10

Social Studies, 07.11.2020 23:10




Mathematics, 07.11.2020 23:10