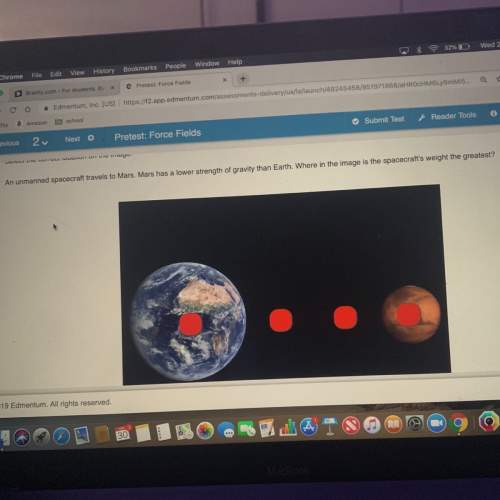
You have two sealed jars of water at the same temperature. in the first jar there is a large amount of water. in the second jar there is a small amount of water. using 3 -4 sentences explain how the vapor pressure of water in the first jar compares with the vapor pressure of water in the second jar.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

You know the right answer?
You have two sealed jars of water at the same temperature. in the first jar there is a large amount...
Questions


Health, 07.06.2021 17:40


Chemistry, 07.06.2021 17:40

Mathematics, 07.06.2021 17:40


Business, 07.06.2021 17:40


History, 07.06.2021 17:40


Mathematics, 07.06.2021 17:40



History, 07.06.2021 17:40



Mathematics, 07.06.2021 17:40