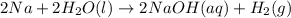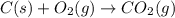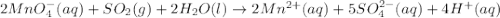Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1


Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
What are the oxidizing agents, and the reducing agents for 2na(aq)+2h2o(l)→2naoh(aq)+h2(g)
c(s...
c(s...
Questions

Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

Arts, 21.11.2020 01:10

English, 21.11.2020 01:10





Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

History, 21.11.2020 01:10

English, 21.11.2020 01:10

Spanish, 21.11.2020 01:10


Chemistry, 21.11.2020 01:10


Mathematics, 21.11.2020 01:10

History, 21.11.2020 01:10