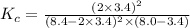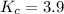Chemistry, 30.01.2020 15:50 sssaaavvvaaagggeee
Suppose 4.2 mol of oxygen and 4.0 mol of no are introduced to an evacuated 0.50-l reaction vessel. at a specific temperature, the equilibrium 2no(g) + o2(g) picture 2no2(g) is reached when [no] = 1.6 m. calculate kc for the reaction at this temperature.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3


Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Suppose 4.2 mol of oxygen and 4.0 mol of no are introduced to an evacuated 0.50-l reaction vessel. a...
Questions











Mathematics, 14.03.2020 00:16





Computers and Technology, 14.03.2020 00:16




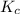 for the reaction is, 3.9
for the reaction is, 3.9 = 4.2 mol
= 4.2 mol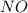 = 4.0 mol
= 4.0 mol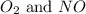 .
.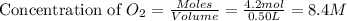
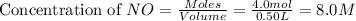
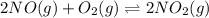
![K_c=\frac{[NO_2]^2}{[NO]^2[O_2]}](/tpl/images/0486/3527/4bf7b.png)
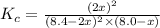 .......(1)
.......(1)