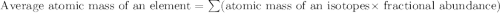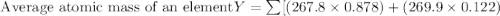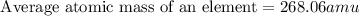Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
Anewly discovered element, y, has two naturally occurring isotopes. 87.8 percent of the sample is an...
Questions


Mathematics, 15.04.2021 04:40



Mathematics, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40


Mathematics, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40


Mathematics, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40


Mathematics, 15.04.2021 04:40

Chemistry, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40

Social Studies, 15.04.2021 04:40



Physics, 15.04.2021 04:40