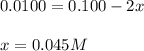10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solution of a substance l. the following equilibrium is established:
m2+(aq) + 2l(aq) picture ml22+(aq)
at equilibrium the concentration of l is found to be 0.0100 mol l–1. what is the equilibrium concentration of ml22+, in mol l–1?
someone me

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

You know the right answer?
10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solu...
Questions

English, 26.09.2019 19:00


Mathematics, 26.09.2019 19:00

Physics, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00

History, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00

History, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00




Mathematics, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00

Geography, 26.09.2019 19:00

Geography, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00

Mathematics, 26.09.2019 19:00

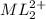 at equilibrium is 0.045 M.
at equilibrium is 0.045 M.![[M^{2+}]_{initial}=0.100M](/tpl/images/0204/7051/8debc.png)
![[L]_{initial}=0.100M](/tpl/images/0204/7051/9e7cd.png)
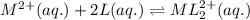
![[L]_{eqllm}=0.0100M](/tpl/images/0204/7051/18682.png)