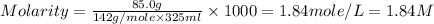Chemistry, 07.01.2020 11:31 lovelyheart5337
What is the molarity of a solution that contains 85.0 grams of na2so4 in 325 milliliters of solution? (the mass of one mole of na2so4 is 142 grams.)
0.195 m
0.599 m
1.84 m
6.22 m

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2


Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

You know the right answer?
What is the molarity of a solution that contains 85.0 grams of na2so4 in 325 milliliters of solution...
Questions




















Social Studies, 18.11.2019 22:31

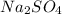 = 85.0 g
= 85.0 g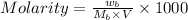
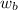 = mass of solute
= mass of solute 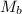 = molar mass of solute
= molar mass of solute 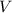 = volume of solution in ml
= volume of solution in ml