Chemistry, 13.12.2019 17:31 jladinosolarsee
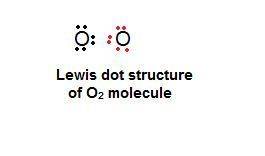
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?
one atom can lose two electrons so that the other atom can gain them and have eight valence electrons.
one atom can lose four electrons to the environment so that a total of eight valence electrons remains.
each atom can share two electrons with the other so that each atom has eight valence electrons.
each atom can lose two electrons so that there is a total of eight valence electrons between the atoms.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?...
Questions




Mathematics, 20.09.2019 16:20







Mathematics, 20.09.2019 16:20






Health, 20.09.2019 16:20


Mathematics, 20.09.2019 16:20

Geography, 20.09.2019 16:20


 molecule is given in the image attached.
molecule is given in the image attached.