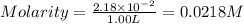Chemistry, 06.10.2019 05:10 officialgraciela67
When zn(oh)2(s) was added to 1.00 l of a basic solution, 1.09×10−2 mol of the solid dissolved. what is the concentration of oh− in the final solution? \

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
When zn(oh)2(s) was added to 1.00 l of a basic solution, 1.09×10−2 mol of the solid dissolved. what...
Questions

History, 03.03.2021 07:40


History, 03.03.2021 07:40

Arts, 03.03.2021 07:40




Mathematics, 03.03.2021 07:40


Mathematics, 03.03.2021 07:40

Mathematics, 03.03.2021 07:40

Mathematics, 03.03.2021 07:40

English, 03.03.2021 07:40

Business, 03.03.2021 07:40


Chemistry, 03.03.2021 07:40

English, 03.03.2021 07:40


History, 03.03.2021 07:40

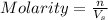
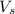 = volume of solution in Liters
= volume of solution in Liters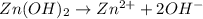
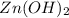 gives = 2 moles of
gives = 2 moles of 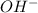
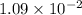 gives =
gives = ![\frac{2}{1}\times 1.09\times 10^{-2}=2.18\times 10^{-2}moles of [tex]OH^-](/tpl/images/0292/2268/6b4cb.png)