
Chemistry, 14.11.2019 19:31 danessaporter
According to the vsepr theory, what is the shape of a molecule that has a central atom bound to three other atoms with no lone pairs of electrons?
a. tetrahedral
b. bent
c. linear
d. trigonal bipyramidal
e. trigonal planar

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
According to the vsepr theory, what is the shape of a molecule that has a central atom bound to thre...
Questions

Geography, 19.12.2019 00:31



Mathematics, 19.12.2019 00:31

Mathematics, 19.12.2019 00:31


History, 19.12.2019 00:31



Mathematics, 19.12.2019 00:31



Mathematics, 19.12.2019 00:31

English, 19.12.2019 00:31

Business, 19.12.2019 00:31

History, 19.12.2019 00:31

History, 19.12.2019 00:31



![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0374/4553/08b6a.png)
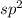 and the electronic geometry of the molecule will be trigonal planar as the electron pairs will repel each other and attain a position which is most stable.
and the electronic geometry of the molecule will be trigonal planar as the electron pairs will repel each other and attain a position which is most stable.


