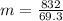Chemistry, 14.10.2019 06:00 cthompson1107
If 832j of energy is required to raise the temperature of a sample of aluminum from 20.0° c to 97.0° c, what mass is the sample of aluminum? (the specific heat of aluminum is 0.90 j/(g × °

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
If 832j of energy is required to raise the temperature of a sample of aluminum from 20.0° c to 97.0°...
Questions





Mathematics, 11.04.2021 15:10

Biology, 11.04.2021 15:10


Mathematics, 11.04.2021 15:20

Mathematics, 11.04.2021 15:20

English, 11.04.2021 15:20



Law, 11.04.2021 15:20

Social Studies, 11.04.2021 15:20


Mathematics, 11.04.2021 15:20

Social Studies, 11.04.2021 15:20


English, 11.04.2021 15:30

Health, 11.04.2021 15:30