Chemistry, 17.12.2019 09:31 dextor1606
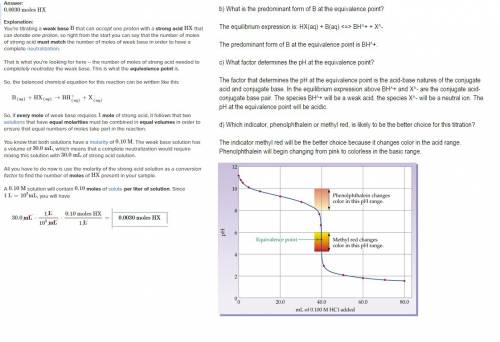
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a 0.10 m solution of a monoprotic strong acid hx. (a) how many moles of hx have been added at the equivalence point? (b) what is the predominant form of b at the equivalence point? (c) what factor determines the ph at the equivalence point? (d) which indicator, phenolphthalein or methyl red, is likely to be the better choice for this titration?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a...
Questions

English, 11.06.2020 05:57


Mathematics, 11.06.2020 05:57


English, 11.06.2020 05:57

History, 11.06.2020 05:57


Mathematics, 11.06.2020 05:57

Biology, 11.06.2020 05:57


Computers and Technology, 11.06.2020 05:57

Mathematics, 11.06.2020 05:57

History, 11.06.2020 05:57





Social Studies, 11.06.2020 05:57

Biology, 11.06.2020 05:57