
An equal number of moles of i2(g) and br2(g) are placed into a closed container and allowed to establish the following equilibrium:
i2(g) + br2(g) 2ibr(g)
keq = 280
which one of the following relates [ibr] to [i2] at equilibrium?
[i2] = [ibr]
[i2] < [ibr]
[i2] = 2 [ibr]
[i2] = 280 [ibr]

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3
You know the right answer?
An equal number of moles of i2(g) and br2(g) are placed into a closed container and allowed to estab...
Questions

Mathematics, 09.04.2021 16:00



Mathematics, 09.04.2021 16:00

Computers and Technology, 09.04.2021 16:00





Mathematics, 09.04.2021 16:00





Chemistry, 09.04.2021 16:00

Biology, 09.04.2021 16:00


History, 09.04.2021 16:00

SAT, 09.04.2021 16:00

Social Studies, 09.04.2021 16:00

![[I_2]](/tpl/images/0244/0546/a6faa.png)
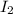 = Moles of
= Moles of 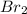
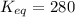
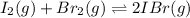
![K_{eq}=\frac{[IBr]^2}{[I_2][Br_2]}](/tpl/images/0244/0546/86934.png)
![[IBr]](/tpl/images/0244/0546/f07e4.png) and
and ![[I_2]](/tpl/images/0244/0546/a1c01.png) .
.![280=\frac{[IBr]^2}{[I_2][I_2]}](/tpl/images/0244/0546/a9dc3.png)
![280=\frac{[IBr]^2}{[I_2]^2}](/tpl/images/0244/0546/b309d.png)
![\sqrt{280}=\frac{[IBr]}{[I_2]}](/tpl/images/0244/0546/a31b5.png)
![[IBr]=16.733\times [I_2]](/tpl/images/0244/0546/79021.png)
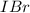 is greater than the concentration of
is greater than the concentration of 

