
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values for free energy and enthalpy at 25.0°c.
g = 130.5 kj/mol
h = 178.3 kj/mol
what is the entropy of the reaction? use g = h – ts.
a. -160.3 j/(mol. k)
b. -47.8 j/(mol. k)
c. 160.3 j/(mol. k)
d. 1,912 j/(mol. k)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3



Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values f...
Questions

Spanish, 18.10.2021 22:20


History, 18.10.2021 22:20





Computers and Technology, 18.10.2021 22:30



Chemistry, 18.10.2021 22:30

English, 18.10.2021 22:30


Mathematics, 18.10.2021 22:30



Computers and Technology, 18.10.2021 22:30

Mathematics, 18.10.2021 22:30


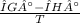
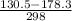 = 0.1603kJ/(mol.K) = 160.3kJ/(mol.K)
= 0.1603kJ/(mol.K) = 160.3kJ/(mol.K) 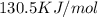
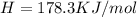
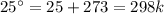
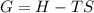
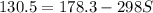

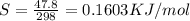
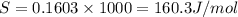 (1KJ=1000J)
(1KJ=1000J)


