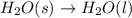The images show two processes. image a: a roasting marshmallow, image b: melting ice. which statement about the two images is true?
1. image a shows a physical change, and image b shows a chemical reaction.
2. image a shows a chemical reaction, and image b shows a physical change.
3. both images show chemical reactions that are endothermic.
4. both images show chemical reactions that are exothermic.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
The images show two processes. image a: a roasting marshmallow, image b: melting ice. which statem...
Questions



Chemistry, 23.06.2019 19:00




Mathematics, 23.06.2019 19:00





English, 23.06.2019 19:00

Social Studies, 23.06.2019 19:00




English, 23.06.2019 19:00

Physics, 23.06.2019 19:00


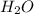 from solid to liquid form and thus is a physical change.
from solid to liquid form and thus is a physical change.