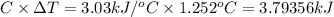Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
When 0.1625 g of magnesium is burned in a bomb calorimeter that has a heat capacity of 3.03 kj/ ∘
Questions


History, 28.06.2020 06:01

Business, 28.06.2020 06:01

Mathematics, 28.06.2020 06:01


Mathematics, 28.06.2020 06:01

Mathematics, 28.06.2020 06:01


Mathematics, 28.06.2020 06:01


Mathematics, 28.06.2020 06:01






History, 28.06.2020 06:01

Mathematics, 28.06.2020 06:01


Mathematics, 28.06.2020 06:01

 = 1.252°C
= 1.252°C