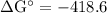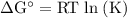Chemistry, 06.01.2020 23:31 daniella0123
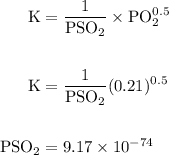
Calcium oxide is used to remove pollutant so2 from smokestack gases. the δg° of the overall reaction is -418.6 kj. what is pso2 in equilibrium with air (po2 = 0.21 atm) and solid cao?
cao(s)+so2 (g)+1⁄2o2 (g)⇔caso4 (s)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

You know the right answer?
Calcium oxide is used to remove pollutant so2 from smokestack gases. the δg° of the overall reaction...
Questions


Mathematics, 22.11.2021 19:20

English, 22.11.2021 19:20

History, 22.11.2021 19:20



Geography, 22.11.2021 19:20




English, 22.11.2021 19:20

Physics, 22.11.2021 19:20

Mathematics, 22.11.2021 19:20


English, 22.11.2021 19:20

English, 22.11.2021 19:20

History, 22.11.2021 19:20

Chemistry, 22.11.2021 19:20

Mathematics, 22.11.2021 19:20

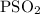 for the given reaction is
for the given reaction is 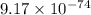 atm.
atm.