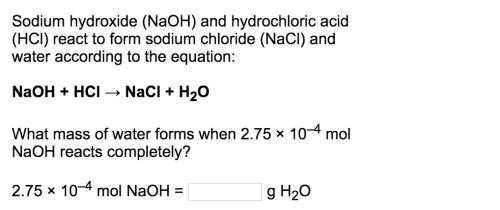
Naoh + hcl → nacl + h2o
what mass of water forms when 2.75 × 10–4 mol naoh reacts completely?<...

Chemistry, 26.08.2019 10:30 jakalenn2018
Naoh + hcl → nacl + h2o
what mass of water forms when 2.75 × 10–4 mol naoh reacts completely?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2



Chemistry, 23.06.2019 12:30
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1
You know the right answer?
Questions

Advanced Placement (AP), 10.12.2020 02:40



Mathematics, 10.12.2020 02:40


Mathematics, 10.12.2020 02:40



Computers and Technology, 10.12.2020 02:40



Chemistry, 10.12.2020 02:40



Physics, 10.12.2020 02:40

Chemistry, 10.12.2020 02:40




Computers and Technology, 10.12.2020 02:40



