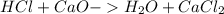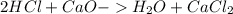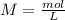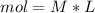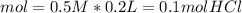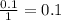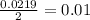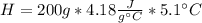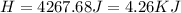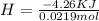During an experiment, a student adds 1.23 g of cao to 200.0 ml of 0.500 m hcl. the student observes a temperature increase of 5.10 °c. assuming the solution\'s final volume is 200.0 ml, the density if 1.00 g/ml, and the heat capacity is 4.184 j/(g·°c, calculate the heat of the reaction, ? hrxn.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1
You know the right answer?
During an experiment, a student adds 1.23 g of cao to 200.0 ml of 0.500 m hcl. the student observes...
Questions



English, 21.07.2019 14:00


Geography, 21.07.2019 14:00

Biology, 21.07.2019 14:00

English, 21.07.2019 14:00


Chemistry, 21.07.2019 14:00

History, 21.07.2019 14:00

Health, 21.07.2019 14:00

Health, 21.07.2019 14:00

History, 21.07.2019 14:00

Chemistry, 21.07.2019 14:00

Biology, 21.07.2019 14:00

Advanced Placement (AP), 21.07.2019 14:00

Advanced Placement (AP), 21.07.2019 14:00


Biology, 21.07.2019 14:00

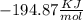
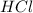 and
and  will produce
will produce  and
and  plus energy, because is an exothermic reaction. The first step is step up the reaction:
plus energy, because is an exothermic reaction. The first step is step up the reaction: