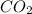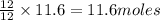In the following reaction, how many liters of carbon dioxide will be produced if
250 liters of...

Chemistry, 27.08.2019 18:00 cassiuspricerules
In the following reaction, how many liters of carbon dioxide will be produced if
250 liters of oxygen is used in the combustion of sucrose, given that both gases are at stp?
c12h22o11 + 12o2 → 12co2 + 11h2o
a. 125 liters b. 500 liters c. 250 liters d. 268.8 liters

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
Questions

English, 16.04.2021 20:10

History, 16.04.2021 20:10


Chemistry, 16.04.2021 20:10

Mathematics, 16.04.2021 20:10

French, 16.04.2021 20:10

Mathematics, 16.04.2021 20:10

Social Studies, 16.04.2021 20:10




Mathematics, 16.04.2021 20:10


Biology, 16.04.2021 20:10

Mathematics, 16.04.2021 20:10



Mathematics, 16.04.2021 20:10

Mathematics, 16.04.2021 20:10

History, 16.04.2021 20:10

 of particles.
of particles.
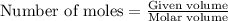
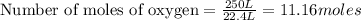
 gives= 12 moles of
gives= 12 moles of