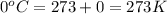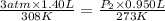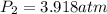Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

You know the right answer?
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0....
Questions


English, 02.12.2020 17:50

Mathematics, 02.12.2020 17:50



Mathematics, 02.12.2020 17:50

Mathematics, 02.12.2020 17:50

Mathematics, 02.12.2020 17:50


Social Studies, 02.12.2020 17:50





Chemistry, 02.12.2020 17:50

Biology, 02.12.2020 17:50


Spanish, 02.12.2020 17:50


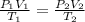
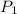 = initial pressure of gas = 3 atm
= initial pressure of gas = 3 atm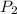 = final pressure of gas = ?
= final pressure of gas = ?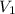 = initial volume of gas = 1.40 L
= initial volume of gas = 1.40 L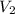 = final volume of gas = 0.950 L
= final volume of gas = 0.950 L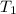 = initial temperature of gas =
= initial temperature of gas = 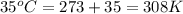
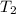 = final temperature of gas =
= final temperature of gas =