

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
Zn(s) + 2hcl(aq) → h2(g) + zncl2(aq) when 25.0 g of zn reacts, how many l of h2 gas are formed at st...
Questions

History, 09.12.2020 14:00

History, 09.12.2020 14:00

Social Studies, 09.12.2020 14:00

Computers and Technology, 09.12.2020 14:00




Social Studies, 09.12.2020 14:00

Computers and Technology, 09.12.2020 14:00

Biology, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00




Mathematics, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

English, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

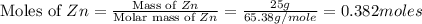
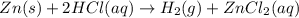
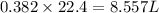 volume of hydrogen gas.
volume of hydrogen gas.

