

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Suppose 13.00 ml of 0.100 m barium hydroxide is required to neutralize 17.00 ml of nitric acid with...
Questions



Mathematics, 29.09.2019 15:30



Mathematics, 29.09.2019 15:30



Mathematics, 29.09.2019 15:30

Social Studies, 29.09.2019 15:30



Mathematics, 29.09.2019 15:30


English, 29.09.2019 15:30

History, 29.09.2019 15:30




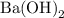 solution is
solution is 
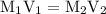
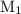 is the molarity of the first solution.
is the molarity of the first solution.
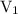 is the volume of the first solution.
is the volume of the first solution.
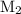 is the molarity of the second solution.
is the molarity of the second solution.
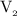 is the volume of the second solution.
is the volume of the second solution.
 . So molarity equation becomes,
. So molarity equation becomes,
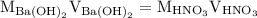 …… (1)
…… (1)
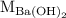 is the molarity of
is the molarity of 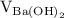 is the volume of
is the volume of 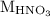 is the molarity of
is the molarity of 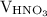 is the volume of
is the volume of 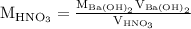 …… (2)
…… (2)



