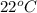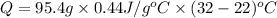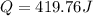Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
The specific heat of nickel is 0.44 j/g*⁰c. how much energy needed to change the temperature of 95.4...
Questions



History, 22.02.2021 22:00



Biology, 22.02.2021 22:00


Mathematics, 22.02.2021 22:00


History, 22.02.2021 22:00

Social Studies, 22.02.2021 22:00


History, 22.02.2021 22:00


Mathematics, 22.02.2021 22:00



History, 22.02.2021 22:00


Mathematics, 22.02.2021 22:00

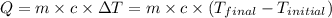
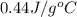
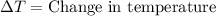
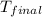 = final temperature =
= final temperature = 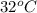
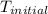 = initial temperature =
= initial temperature =