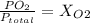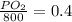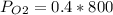Chemistry, 13.11.2019 09:31 blueflu5120
Amixture of gases with a pressure of 800.0 mm hg contains 60% nitrogen and 40% oxygen by volume. what is the partial pressure of oxygen in this mixture?
a. 140.0 mm hg
b. 320.0 mm hg
c. 373.0 mm hg
d. 480.0 mm hg

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1


Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
Amixture of gases with a pressure of 800.0 mm hg contains 60% nitrogen and 40% oxygen by volume. wha...
Questions

Mathematics, 12.02.2021 06:50



Mathematics, 12.02.2021 06:50


Chemistry, 12.02.2021 06:50

Mathematics, 12.02.2021 06:50



Mathematics, 12.02.2021 06:50

Health, 12.02.2021 06:50


Computers and Technology, 12.02.2021 06:50




Mathematics, 12.02.2021 06:50

Biology, 12.02.2021 06:50


Mathematics, 12.02.2021 06:50

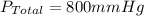
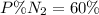
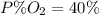
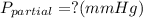
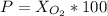
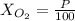
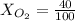
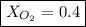
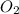 :
: