
Chemistry, 27.12.2019 18:31 chandranewlon
What is the maximum amount of moles of p2o5 that can theoretically be made from 136 g of p4 and excess oxygen

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1
You know the right answer?
What is the maximum amount of moles of p2o5 that can theoretically be made from 136 g of p4 and exce...
Questions

Mathematics, 30.06.2019 07:10

Mathematics, 30.06.2019 07:10



Mathematics, 30.06.2019 07:10




Mathematics, 30.06.2019 07:10

Mathematics, 30.06.2019 07:10

History, 30.06.2019 07:10

History, 30.06.2019 07:10

Mathematics, 30.06.2019 07:10



History, 30.06.2019 07:10


Biology, 30.06.2019 07:10

History, 30.06.2019 07:10

 theoretically made is 2.194 moles.
theoretically made is 2.194 moles.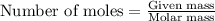
 = 136 g
= 136 g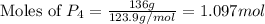
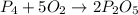
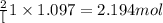 of
of 


