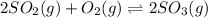Consider the reaction.
2so2(g)+o2(g) < > 2so3(g)
when does the given...

Consider the reaction.
2so2(g)+o2(g) < > 2so3(g)
when does the given chemical system reach dynamic equilibrium?
when the forward and reverse reactions stop
when the rate of the forward reaction is higher than the rate of the reverse reaction
when the concentration of the reactants is higher than the concentration of the products
when the rates of the forward and reverse reactions are equal

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Questions

Mathematics, 15.01.2020 23:31


English, 15.01.2020 23:31

History, 15.01.2020 23:31


Mathematics, 15.01.2020 23:31

Arts, 15.01.2020 23:31


Mathematics, 15.01.2020 23:31


History, 15.01.2020 23:31

Mathematics, 15.01.2020 23:31

Mathematics, 15.01.2020 23:31






Mathematics, 15.01.2020 23:31