

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3


Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2
You know the right answer?
Ammonia, nh3 (hf = –46.2 kj), reacts with oxygen to produce water (hf = –241.8 kj) and nitric oxide,...
Questions


Mathematics, 18.03.2021 02:50

English, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Geography, 18.03.2021 02:50

English, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50

History, 18.03.2021 02:50

Chemistry, 18.03.2021 02:50

Computers and Technology, 18.03.2021 02:50


Social Studies, 18.03.2021 02:50


Social Studies, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

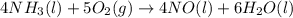
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0331/3324/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{NO}\times \Delta H_{NO})]-[(n_{NH_3}\times \Delta H_{NH_3})+(n_{O_2}\times \Delta H_{O_2})]](/tpl/images/0331/3324/c1447.png)
![\Delta H=[(6\times -241.8)+(4\times 91.3)]-[(4\times -46.2)+(5\times 0)]\\\\\Delta H=-900.8KJ](/tpl/images/0331/3324/e4f51.png)


