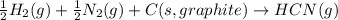Chemistry, 29.01.2020 00:51 marialandingin7520
Write a balanced chemical equation for the standard formation reaction of gaseous hydrogen cyanide hcn .

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Write a balanced chemical equation for the standard formation reaction of gaseous hydrogen cyanide h...
Questions

Social Studies, 22.04.2020 20:54




Computers and Technology, 22.04.2020 20:54


Computers and Technology, 22.04.2020 20:54

Chemistry, 22.04.2020 20:54

English, 22.04.2020 20:54



Mathematics, 22.04.2020 20:54

Mathematics, 22.04.2020 20:54


Mathematics, 22.04.2020 20:54

History, 22.04.2020 20:54

Mathematics, 22.04.2020 20:54

French, 22.04.2020 20:54


Social Studies, 22.04.2020 20:54