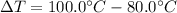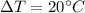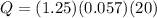Chemistry, 01.09.2019 14:00 hardwick744
How much heat, in calories, is given off when 1.25 grams of silver is cooled from 100.0°c to 80.0°c? (the specific heat of silver is 0.057 cal/g°c)
1.43 cal
0.0713 cal
5.70 cal
7.13 cal

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
How much heat, in calories, is given off when 1.25 grams of silver is cooled from 100.0°c to 80.0°c?...
Questions









Mathematics, 18.12.2019 22:31


Biology, 18.12.2019 22:31


Computers and Technology, 18.12.2019 22:31


Engineering, 18.12.2019 22:31



Mathematics, 18.12.2019 22:31


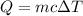
 is the change in temperature.
is the change in temperature.