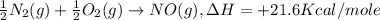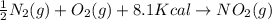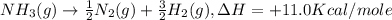Chemistry, 09.01.2020 06:31 MysteryDove12
Select all of the following reactions that are endothermic.
-h2(g) + ½o2(g) → h2o(g), δh = -57.83 kcal/mole
-½n2(g) + ½o2(g) → no(g), δh = +21.6 kcal/mole
-½n2(g) + o2(g) + 8.1 kcal → no2(g)
-½n2(g) + 3/2h2(g) → nh3(g) + 11.0 kcal/mole
-nh3(g) → ½n2(g) + 3/2h2(g), δh = +11.0 kcal/mole

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

You know the right answer?
Select all of the following reactions that are endothermic.
-h2(g) + ½o2(g) → h2o(g), δh = -57...
-h2(g) + ½o2(g) → h2o(g), δh = -57...
Questions

Mathematics, 12.04.2021 22:00

Mathematics, 12.04.2021 22:00







Mathematics, 12.04.2021 22:00


Mathematics, 12.04.2021 22:00


Mathematics, 12.04.2021 22:00


History, 12.04.2021 22:00

Mathematics, 12.04.2021 22:00


Mathematics, 12.04.2021 22:00