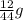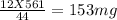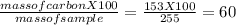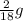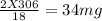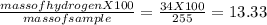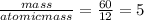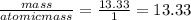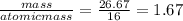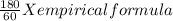Chemistry, 26.01.2020 16:31 jaymee2904p88tgh
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o, and you find that 561 mg of co2 is produced, along with 306 mm of h2o.
if the substance contains only c, h, and o, what is the empirical formula
if the molar mass of the compound is 180 g/mol what is the molecular formula of the compound

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o,...
Questions

Physics, 05.02.2020 10:43

Mathematics, 05.02.2020 10:43


Health, 05.02.2020 10:43






Computers and Technology, 05.02.2020 10:43




Mathematics, 05.02.2020 10:43

Mathematics, 05.02.2020 10:43


History, 05.02.2020 10:43



Mathematics, 05.02.2020 10:43