
Avogadro’s law and charles’s law describe a proportionality of the volume of a gas when the pressure is constant. which describes the proportionality that allows these laws to be combined when describing a gas?
volume is directly proportional to temperature (avogadro’s law) and to moles (charles’s law).
volume is inversely proportional to temperature (avogadro’s law) and to moles (charles’s law).
volume is directly proportional to moles (avogadro’s law) and to temperature (charles’s law).
volume is inversely proportional to moles (avogadro’s law) and to temperature (charles’s law).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
Avogadro’s law and charles’s law describe a proportionality of the volume of a gas when the pressure...
Questions



History, 19.07.2019 04:00


History, 19.07.2019 04:00


Chemistry, 19.07.2019 04:00

Biology, 19.07.2019 04:00


History, 19.07.2019 04:00






English, 19.07.2019 04:00


History, 19.07.2019 04:00


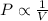 (At constant temperature and number of moles)
(At constant temperature and number of moles)
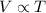 (At constant pressure and number of moles)
(At constant pressure and number of moles)
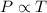 (At constant volume and number of moles)
(At constant volume and number of moles)
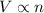 (At constant temperature and pressure)
(At constant temperature and pressure)

